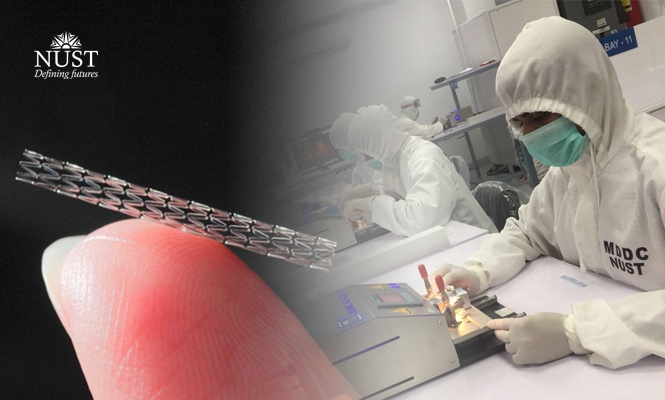
Pakistan’s health indicators severely fall short of the required levels. Per capita expenditure on health is very low in Pakistan and in the last eleven years has not increased significantly.Later in 2019 National University of Sciences and Technology amplified the healthcare sector by being the first Pakistani university to develop low-cost indigenous and implants including Bare Metal Stents (BMS), Drug Eluting Stents (DES) and Percutaneous Transluminal Coronary Angioplasty Balloon Catheters (PTCA balloon catheters). In light of this, the Government of Pakistan has funded NUST with up to Rs. 331 Million for the development of Medical Devices Development Center, MDDC.
NUST RECEIVES MANUFACTURING LICENCE FOR MEDICAL DEVICES FROM DRAP
-Share this story:
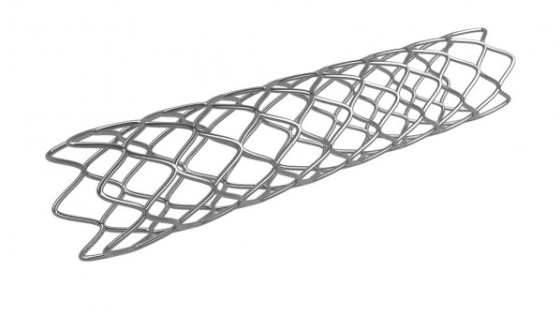
MDDC at NUST has developed bare metal stents, angioplasty balloon catheters, and has conducted various studies, clinical trials and analysis in Pakistan and abroad. MDDC will gain a stronger foothold in the industry after various studies, clinical trials, and market analysis to meet/surpass the high standards set by international standards. The production of the cost-effective stents will allow MDDC to establish a strong presence not only locally but globally as well.
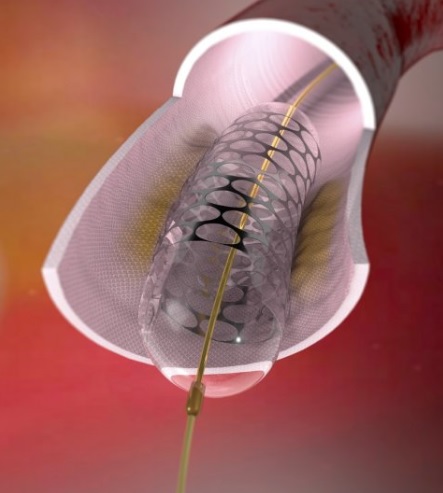
In latest development, the National University of Sciences & Technology (NUST) has secured the manufacturing license from Pakistan’s Drug Regulator Authority (DRAP) for the mass production of Cardiac Stents and Angioplasty Balloon Catheters. This license is a mandatory requirement to manufacture any medical device in Pakistan which is obtained after critical inspection of the facility by the team of inspectors appointed by DRAP. Under this license, NUST is authorized to manufacture the medical devices, especially the stents to be produced commercially for 1/5th price of those available internationally and these will perform on par with other stents manufactured by foreign multi-national businesses. As far as the product technology, raw material and designs are concerned, MDDC has acquired these from Germany, our affiliate (industrial partner). The validation of the finished products, developed at MDDC has been carried out by the German affiliate partner industry.
Medical Devices Development Center (MDDC) at NUST is a dedicated and certified facility in two areas:
- To produce Bare Metal Stent System (BMS) and Percutaneous Coronary Angioplasty Catheters (PTCA) at a lower cost for the cardiology hospitals in Pakistan
- To produce Drug Eluting Stent System (DES) which covers a larger market share and is more costly or almost inaccessible for the poor people of Pakistan.
Objectives:
- Ensure availability of BMS, PTCA and DES to the poor and save foreign exchange being spent every year to import stents.
- To equip this Center to meet the market demand of BMS and DES systems throughout the country.
Benefits to Society:
- There is an enormous quantitative financial impact involved by indigenization of biomedical devices which are frequently used for the treatment of general public of Pakistan.
- Employment generation
- Low cost treatment solution for the patient suffering from Heart (cardiac) diseases
- Opportunity for the students to experience Product Realization (from its inception to completion)
- Trend setting for indigenization of medical devices.
Special Assistant to PM on Health Dr. Zafar Mirza lauded the efforts of NUST and showing pleasure at such an innovative development he said, the production of heart stents domestically as per the international standards, will help in reducing its prices, facilitate the patients and ultimately will leave positive impact on the country.
Rector NUST, Lt Gen Naweed Zaman, HI (M), (Retd) applauded the efforts of NUST for having achieved such a great milestone as the solution by NUST has been approved in the international market by adhering to Good Manufacturing Practices (GMP) and also conforming to regulatory requirements of Drug Regulatory Authority of Pakistan (DRAP) and Medical Health Regulatory Authority (MHRA), United Kingdom (for its both ISO 13485 Certification and Product registration/ISO and DRAP Certification).
MDDC will continue to work on and develop such technologies, designed to help physicians improve patient care and ensure better health outcomes.This will be a trend setting initiative in a country like Pakistan so that other industrial groups operating in Pakistan will follow the same trail and revolutionize the medical device industry in this country.
The author works as anAssociate Professor at the Department of Biomedical Engineering and Sciences, School of Mechanical and Manafacturing Engineering (SMME), NUST, and can be reached at [email protected]